Abstract
Introduction: GRIFFIN (NCT02874742) is a multicenter, randomized, open-label, active-controlled, phase 2 study comparing daratumumab in combination with bortezomib, lenalidomide and dexamethasone (D-RVd) vs RVd in transplant-eligible patients (pts) with newly diagnosed multiple myeloma (NDMM). In the primary analysis (median follow-up: 13.5 months), D-RVd improved the stringent complete response rate by end of post-autologous stem cell transplant (ASCT) consolidation; the rates of minimal residual disease (MRD) negativity (10-5) were also improved for D-RVd vs RVd at median follow-up of 22.1 months. With longer follow-up after all pts completed 2 years of maintenance therapy (median: 38.6 months), D-RVd continued to induce deeper responses and improve MRD negativity (10−5) rates vs RVd. Here, we present the patient-reported outcomes (PROs) from GRIFFIN at the final study analysis after all pts completed 1 year of follow-up post maintenance therapy with 49.6 months of median follow-up.
Methods: Pts with NDMM who were eligible for ASCT were randomized to receive 4 cycles of D-RVd or RVd induction and ASCT, followed by 2 cycles of D-RVd or RVd consolidation, and D-R or R maintenance for up to 24 months. Pts could continue on R maintenance after study treatment completion per local standard of care. PROs were assessed using the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Core 30-item (EORTC QLQ-C30), EORTC QLQ Multiple Myeloma Module 20-item (EORTC QLQ-MY20), and EuroQol 5-dimensional (EQ-5D-5L) descriptive system. Questionnaires were completed at baseline (BL), on day 1 of cycles (C) 2, 3, 5, on day 21 of C4, post-ASCT consolidation, and after months 6, 12, 18 and 24 of maintenance. Analyses were conducted on all randomized pts. Compliance with PRO assessments was calculated by dividing the number of assessments received by the number of assessments expected at each time point. Time to first worsening, hazard ratios (HR), and associated 95% confidence intervals (CI) were estimated using Kaplan- Meier methods and Cox proportional hazards model. A distribution-based method was used to define worsening in scores. Mean change from BL was analyzed using a mixed-effects model with repeated measures.
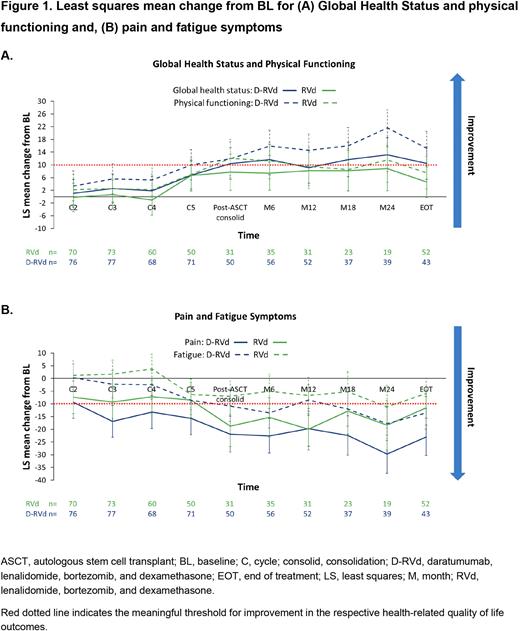
Results: A total of 207 pts were randomized (D-RVd, n=104; RVd, n=103). Compliance rates for all PRO questionnaires were >81% at BL, were 62.6% in the D-RVd group and 48.6% in the RVd group post-ASCT consolidation and decreased with follow-up during the maintenance phase (maintenance Month 24: D-RVd, 49.4%; RVd, 45.1%). Mean BL values for QLQ-C30 Global Health Status (GHS), functional and symptom subscales and EQ-5D-5L Visual Analog Scale (VAS) were comparable between the D-RVd and RVd groups. Least squares (LS) mean change from BL improved for GHS and physical functioning during the maintenance phase in the D-RVd group (Figure1A) and was numerically greater with D-RVd vs RVd (maintenance Month 24, LS mean change [95% CI] for GHS: 13.2 [7.9, 18.5] vs 8.8 [1.9, 15.6]; LS mean change [95% CI] for physical functioning: 21.6 [16.1, 27.2] vs 11.6 [4.5, 18.6)], P=0.0158). Reduction in pain symptoms was seen at most time points for pts in both treatment arms, with a large reduction (≥20-point change) in pain in favor of D-RVd at post-ASCT consolidation and throughout the maintenance phase (maintenance Month 24 [LS mean change {95% CI}: -29.7 {-37.3, -22.2} vs -18.4 {-28.1, -8.7}; P=0.0497; Figure 1B). Pts treated with D-RVd experienced a greater reduction in fatigue symptoms at maintenance Month 6 than those treated with RVd (LS mean change [95% CI]: -13.5 [-19.4, -7.6] vs -5.0 [-11.8, 1.7]; P=0.0347; Figure 1B). Similarly, EQ-5D-5L VAS was higher with D-RVd vs RVd at maintenance Month 18. Improvements from BL were seen in EORTC QLQ MY20 disease symptoms and future perspective for both treatment groups and generally favored D-RVd at several time points. Median time to first worsening in GHS was 45.2 months vs 14.4 months with D-RVd vs RVd (HR [95% CI]: 0.71 [0.46, 1.10]).
Conclusions: The addition of daratumumab to RVd resulted in greater improvements in health-related quality of life (HRQoL) for pts who continued on maintenance treatment post-ASCT consolidation vs RVd alone, with a notable reduction in pain symptoms. Overall, these findings further support the addition of daratumumab to RVd in transplant-eligible pts with NDMM without compromise of HRQoL.
Disclosures
Silbermann:Janssen: Membership on an entity's Board of Directors or advisory committees; Sanofi-Aventis: Membership on an entity's Board of Directors or advisory committees, Research Funding. Kaufman:AbbVie, Genentech, and Bristol Myers Squibb: Consultancy; AbbVie: Other: Member of steering committee; Incyte: Other: Member of data safety monitoring committee . Sborov:Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Sanofi: Consultancy; BMS: Consultancy; Abbvie: Consultancy; GSK: Consultancy, Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy; Bioline: Consultancy; Pfizer: Consultancy. Reeves:Incyte, BMS, PharmaEssentia, CTI Biopharma: Honoraria; Hemostasis & Thrombosis Research Society Mentored Research Award sponsored by CSL Behring: Research Funding. Rodriguez:Janssen, BMS, Takeda, AbbVie, karyopharm, Artiva: Consultancy, Speakers Bureau. Chari:Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Seattle Genetics: Membership on an entity's Board of Directors or advisory committees, Research Funding; Array Biopharma: Research Funding; Glaxo Smith Klein: Research Funding; Novartis Pharmaceuticals: Research Funding; Oncoceutics: Research Funding; Pharmacyclics: Research Funding; Karyopharm: Consultancy, Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb: Consultancy; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Sanofi: Membership on an entity's Board of Directors or advisory committees. Costa:Sanofi: Consultancy, Honoraria; Genentech: Research Funding; AbbVie: Research Funding; Adaptive Biotechnologies: Consultancy, Honoraria; Bristol Myers Squibb: Consultancy, Honoraria, Research Funding; Amgen: Consultancy, Honoraria, Research Funding; Janssen: Consultancy, Honoraria, Research Funding. Anderson:AbbVie: Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Sanofi: Honoraria, Membership on an entity's Board of Directors or advisory committees; Beigene: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pharmacyclics: Honoraria, Membership on an entity's Board of Directors or advisory committees; GSK: Honoraria, Membership on an entity's Board of Directors or advisory committees; Karyopharm: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Prothena: Honoraria, Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees. Shah:GSK, Amgen, Indapta Therapeutics, Sanofi, CareDx, Kite, Karyopharm, Oncopeptides,: Consultancy; AstraZeneca: Current Employment, Current equity holder in publicly-traded company; Aztra Zeneca: Current Employment, Other: stock ownership; Celgene/BMS, Janssen, Bluebird Bio, Sutro Biopharma, Teneobio, Poseida, Nektar, Precision Biosciences: Research Funding. Bumma:Sanofi, Genzyme: Other: Ad Board, Speakers Bureau; Amgen: Consultancy, Speakers Bureau; Janssen: Membership on an entity's Board of Directors or advisory committees, Other: Ad Board. Holstein:BMS/Celgene: Consultancy, Research Funding; Janssen: Consultancy, Research Funding; Oncopeptides: Consultancy, Research Funding; Sanofi: Consultancy; GSK: Consultancy, Research Funding; Takeda: Consultancy, Research Funding; Secura Bio: Consultancy; Genentech: Consultancy. Costello:BMS, Takeda, Janssen, Pfizer: Honoraria, Research Funding. Jakubowiak:Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees. Orlowski:Asylia Therapeutics, Inc.: Current equity holder in private company; Abbvie, BioTheryX, Inc., Bristol-Myers Squibb, Janssen Biotech, Karyopharm Therapeutics, Inc., Meridian Therapeutics, Monte Rosa Therapeutics, Neoleukin Corporation, Oncopeptides AB, Regeneron Pharmaceuticals, Inc., Sanofi-Aventis, and Takeda Pharmaceutic: Honoraria, Membership on an entity's Board of Directors or advisory committees; CARsgen Therapeutics, Celgene/Bristol Myers Squibb, Exelixis, Janssen Biotech, Sanofi-Aventis, Takeda Pharmaceuticals North America, Inc.: Research Funding; Asylia Therapeutics, Inc., BioTheryX, Inc., Heidelberg Pharma, Inc.: Research Funding. Shain:GSK, Janssen and BMS and speaker's bureau for GSK, BMS, Sanofi, Karyopharm, Takeda, Janssen, Adaptive and Amgen: Other: Advisory Committee; Bristol Myers Squibb (BMS), Janssen, GlaxoSmithKline (GSK), Adaptive, Sanofi, and Takeda, and Amgen: Honoraria; AbbVie, Karyopharm: Research Funding; Janssen,BMS: Other: PI of clinical trials. Cowan:Harpoon: Research Funding; Sanofi-Aventis: Research Funding; BMS: Consultancy, Research Funding; Nektar: Research Funding; AbbVie: Consultancy, Research Funding; Allogene: Consultancy; EUSA: Consultancy; GSK: Consultancy; Adaptive Biotechnologies: Membership on an entity's Board of Directors or advisory committees; Secura bio: Consultancy; Janssen: Consultancy, Research Funding. Gries:Janssen Pharmaceutical: Current Employment, Current holder of stock options in a privately-held company. Pei:Janssen: Current Employment, Current equity holder in publicly-traded company. Cortoos:Janssen: Current Employment, Current equity holder in publicly-traded company. Patel:Companies of Johnson & Johnson: Current Employment, Current equity holder in publicly-traded company. Lin:Janssen: Current Employment, Current holder of stock options in a privately-held company. Richardson:Takeda: Consultancy, Research Funding; Takeda: Research Funding; Takeda, Abbvie, GSK, and Celgene: Consultancy; Protocol Intelligence: Consultancy; AstraZeneca: Consultancy; Regeneron: Consultancy; GlaxoSmithKline: Consultancy; Secura Bio: Consultancy; Sanofi: Consultancy; Karyopharm: Consultancy, Research Funding; Takeda, Celgene, and GSK: Honoraria; Oncopeptides: Consultancy, Research Funding; Celgene/BMS: Consultancy, Research Funding; Takeda and GSK: Other: Travel expenses from Takeda and GSK. Usmani:Abbvie, Amgen, BMS, Celgene, EdoPharma, Genentech, Gilead, GSK, Janssen,Oncopeptides, Sanofi, Seattle Genetics, SecuraBio, SkylineDX, Takeda, TeneoBio: Consultancy; Amgen, BMS, Janssen, Sanofi: Speakers Bureau; Amgen, Array Biopharma, BMS, Celgene, GSK, Janssen, Merck, Pharmacyclics, Sanofi, Seattle Genetics, SkylineDX, Takeda: Research Funding. Voorhees:Abbvie, Amgen, BMS, GSK, Karyopharm, Novartis, Oncopeptides, Pfizer, Sanofi, SecuraBio: Consultancy, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal